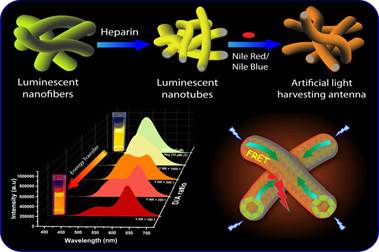
Inspired by natural photosynthetic systems, researchers have developed a new method of harvesting artificial light using organic nanotubes, which can be utilized in solar cells, photocatalysis, optical sensors, and tunable multi-color light-emitting materials.
In nature, plants and photosynthetic bacteria capture sunlight and deliver it to the reaction center through a cascade of energy and electron transfer steps for its eventual storage in the form of chemical energy. The antenna chromophores in the light-harvesting complexes are precisely aligned into arrays by the surrounding proteins, which in turn allows the energy migration between them in a highly efficient manner. Mimicking natural photosynthetic systems and understanding the fundamental processes of energy transfer has gained enormous interest in recent years, especially for systems that need energy conversion and storage.
Towards this direction, Dr. Supratim Banerjee from the Indian Institute of Science Education and Research (IISER) Kolkata, an autonomous Institute under the Ministry of Education, and Dr. Suman Chakrabarty from the S. N. Bose National Center for Basic Sciences (SNBNCBS), Kolkata, an autonomous institute of Department of Science and Technology (DST) carried out experimental and computational investigations on artificial light-harvesting in organic nanotubes derived from the union of an organic fluorescent molecule and a therapeutically important biopolymer. The former is an amphiphilic cationic molecule called cyano stilbenes (an organic molecule with fluorescent properties that are known to exhibit enhanced emission in their aggregated state), and the latter is an anionic therapeutically important bio-polymer called heparin (used as an anti-coagulant-during-surgery-and-in-post-operative-treatments) in aqueous media.
In the presence of heparin, the cationic cyano stilbenes employed in this study formed nanotubes with bright greenish-yellow emission through an electrostatically driven co-assembly process. Just like the antenna chromophores or pigmented (coloured) membrane-associated vesicles used to perform photosynthesis in bacteria, the nanotubes acted as highly efficient energy donors (antennae) in a system that mimicked the natural photosynthetic process.
They donated energy to acceptor dyes such as Nile Red and Nile Blue, resulting in emission color tuning from initial greenish-yellow to orange-red, including white light. The energy transfer phenomenon demonstrated in this study is known as FRET (Förster resonance energy transfer), which has significant importance in different applications such as the determination of DNA/RNA structures, mapping biological membranes, real-time PCR tests, and so on. The future is moving towards the conversion of solar energy for storage as chemical or electrical energy, and the process of energy transfer is a key factor for such applications.
In the study published in Chemical Science, the flagship journal of the Royal Society of Chemistry, the formation of the nanotubes was investigated by employing absorption and fluorescence spectroscopy, transmission electron microscopy (TEM), and fluorescence lifetime imaging microscopy (FLIM) studies. Molecular Dynamics (MD) simulation studies demonstrated that the cyano stilbene molecules formed cylindrical structures in the presence of heparin. The local molecular level interactions and packing of the cyano stilbene chromophores that led to the formation of one-dimensional nanostructures were also visualized and quantified through the simulation studies. Due to the temperature responsiveness of the FRET process in these systems, they were further employed as ratiometric emission thermometers (that sense temperature based on the variation in emission intensity at two different wavelengths) in the temperature range 20–90 °C, and this highlighted a practical application of these artificial light-harvesting systems.